Our research
The research of our group is focused on development of synthetic methodologies for solution as well as solid-phase synthesis. The known as well as developed synthetic protocols are then used for preparation of compounds of desired properties. The main attention is paid to anticancer agents and molecular diagnostics. The best results of last few years are listed here.
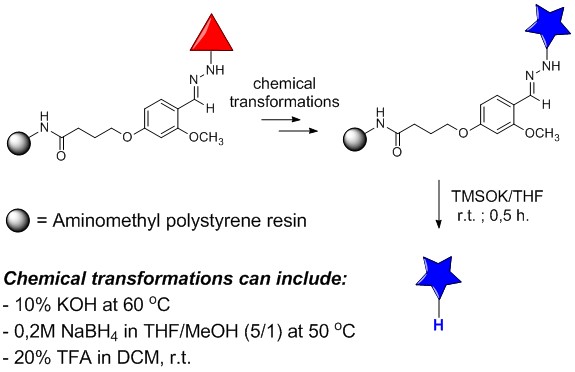
For a long time our research group is focused on application of solid-phase synthesis for preparation of various organic compounds via combinatorial manner. For the purposes of preparation of compounds under various different conditions during the reaction sequence we have developed quite new hydrazone linker stable in acids, common bases, hydrides, etc even at elevated temperature. Linker is selectivelly cleavable by trimethylsilanolate for releasing of final compound from resin.
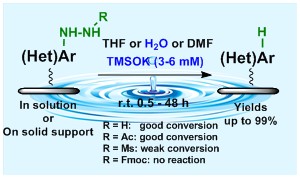
Within our research activities we discovered "magic" properties of potassium trimethylsilanolate, which can serve for very gentle removal of hydrazinyl group from aromatic substrates. The reaction proceeds not only in organic solvents, but surprisingly also in water. Whenever hydrazingroup should be preserved during other trimethylsilanolate application, the protection of Fmoc group can be applied. The methodology is applicable not only in solution, but also for polymer supported reactions.
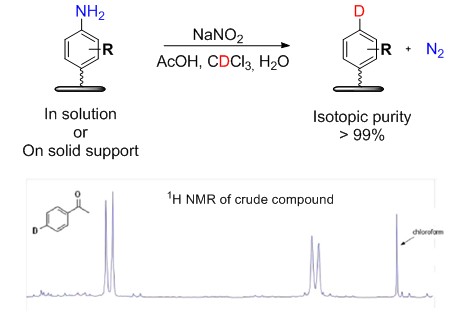
Recently we have developed an easy methodology for deuteration of aromatic compounds. Simple treatment of aromatic amines with deuterated chloroform, water and sodium nitrite causes replacement of amino group by deuterium. The reaction is fast and isotopic occupation is quantitative. The deuterium implementation can be done quantitativelly in solution and less selectivelly on polymer supported substrate.

A synthetic three-fluorophore system with two enzymatically cleavable
linkers has been developed for the simultaneous detection of two
proteases in a mixture. The probe was designed to afford single
excitation/triple emission ratiometric detection through a fluorescence
change during the cleavage of a peptide linker. The developed assays
were verified for trypsin and chymotrypsin as the model enzymes.
We were also awarded by a possibility to create Cover page. The creation made by Yana Okorochenkova is figured above.
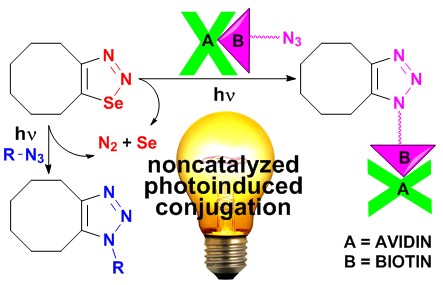
Among the conjugation reactions the non-catalysed ones maintain significant position in research of bioorthogonal reactions. We found out that the selenadiazoles can be used in photoinitiated conjugations of biomolecules with small molecular entities. The reaction can be used for a chemoselective labelling of biomolecules. Reaction was verified for labelling of modified avidine as a proteine representative.
In the article we have studied the photoreduction of bromoalkenes with use of 1,3-dimethyl-2-phenyl-1H-benzo-[d]imidazoline (DMBI)
and 9,10-dicyanoanthracene. With deuterated DMBI analogs (the most effective was DMBI-d11),
satisfactory to excellent isotopic yields were reached. DMBI-d11
could also be regenerated from reaction mixtures by up to 50%. The combination
of the photodebromination reaction with conventional methods for bromoalkenes
synthesis enables sequential monodeuteration of double bond without necessity
of a metal catalyst. The proces is based on eco-friendly approach.